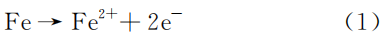Abstract: A company's Q245R steel boiler tube experienced a horizontal tube burst after only 8 months of use. The cause of the boiler tube burst was analyzed using macroscopic observation, chemical composition analysis, hardness testing, metallographic inspection, scanning electron microscopy, X-ray diffraction, and other methods. The results show that the water vapor passing through the Q245R steel
boiler tube contained many impurities, such as NaCl and Na₂CO₃, which caused the pipe corner to become blocked and narrowed, leading to abnormal steam flow rate and temperature in the front section of the pipe. Due to high temperature, high pressure, and corrosive media, the inner wall of the tube continuously oxidized, causing the tube wall to thin and decarburize. This significantly reduced the tube’s strength, making it insufficient to withstand the working pressure, ultimately leading to the tube burst.
Q245R steel is primarily used to manufacture boiler and pressure vessel components, such as low-temperature superheaters, reheaters, economizers, and water-cooled walls. Since carbon steel will graphitize when operated for extended periods above 450°C, the maximum long-term operating temperature of the heating surface tube should preferably remain below 450°C. Within this temperature range, the strength of Q245R steel meets the requirements of superheaters and steam pipes, and it exhibits good oxidation resistance, toughness, welding performance, and more. Its cold and hot processing performances are excellent.
A company's Q245R steel boiler tube experienced a horizontal tube burst after 8 months of use. The tube burst occurred in the straight section of the tube just before a bend. The medium within the boiler tube is water vapor, with a working temperature of 400°C, a working pressure of 1.2MPa, and a design tube wall thickness of 4.5 mm. There is no high-temperature outside the pipe fittings. To determine the cause of the Q245R steel boiler tube failure, a series of tests and analyses were conducted to prevent similar incidents in the future.
Boiler tubes from the burst location and bend location were selected for analysis, as shown in Figure 1. The No. 1 and No. 2 sample boiler tubes had bursts, while the No. 3 and No. 4 samples showed no burst. Samples No. 2 and No. 4 were cut horizontally, revealing that the No. 2 tube was hollow, with a severely corroded inner wall. The tube wall had black and white discoloration, with the outer white ring being thinner and the inner ring gray-black. In contrast, the white tube wall of sample No. 4 was thicker, and the tube was filled with black blockages, as shown in Figure 2.

Figure 1 Macromorphology of boiler tubes and sample numbers

Figure 2 Cross-sectional macromorphology of samples No. 2 and No. 4
The chemical composition of the outer walls of samples No. 2 and No. 4 was analyzed using the ARL-4460 direct reading spectrometer, with the results shown in Table 1. Table 1 shows a significant difference in the carbon content between the two samples. The carbon content of the outer wall of sample No. 2 is only 0.018%, while that of sample No. 4 is 0.16%. The content of other elements shows little difference.
Table 1 Chemical composition of boiler tubes
| Items |
C |
Si |
Mn |
P |
S |
| Sample No. 2 |
0.018 |
0.21 |
0.53 |
0.010 |
0.003 |
| Sample No. 4 |
0.16 |
0.22 |
0.52 |
0.011 |
0.003 |
| Standard Value |
≤0.20 |
≤0.35 |
0.50-1.10 |
≤0.025 |
≤0.01 |
After grinding and polishing the cross sections of samples No. 2 and No. 4, the hardness was tested using a KB30S Vickers hardness tester. The hardness of the outer wall of sample No. 2 was measured. The results are presented in Table 2. As presented in Table 2, the hardness of sample No. 2 is 90 HV10, while the hardness of sample No. 4 is 185 HV10, demonstrating a significant difference between the two.
Table 2 Hardness test results of the boiler tubes
| Items |
Test Value (HV10) |
Average Value |
| Sample No. 2 |
90, 91, 90 |
90 |
| Sample No. 4 |
188, 185, 183 |
185 |
The outer wall of sample No. 2 and the longitudinal section of sample No. 4 were inlaid and polished sequentially, and then examined for non-metallic inclusions using an Axio Imager M2m optical microscope. The non-metallic inclusions are presented in Table 3 and Figure 3. It can be observed that the B-type inclusions in sample No. 2 are rated at 1.0, while the B-type inclusions in sample No. 4 are rated at 2.0.
Table 3 Rating results of non-metallic inclusions in boiler tubes
| Sample |
Type A Coarse Series |
Type A Fine Series |
Type B Coarse Series |
Type B Fine Series |
Type C Coarse Series |
Type C Fine Series |
Type D Coarse Series |
Type D Fine Series |
DS |
| Sample No. 2 |
0.0 |
0.0 |
0.0 |
1.0 |
0.0 |
0.0 |
0.5 |
0.5 |
0.0 |
| Sample No. 4 |
0.0 |
0.0 |
0.0 |
2.0 |
0.0 |
0.0 |
0.5 |
0.5 |
0.0 |
a) sample No 2 b) sample No 4
Figure 3 Morphology of nonmetallic inclusions of the boiler tubes
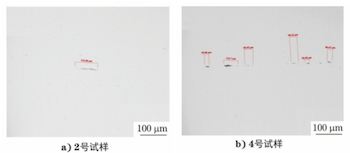
The cross sections of samples No. 2 and No. 4 were taken, inlaid, polished, and etched sequentially, and their polished and microstructural morphologies were observed using an Axio Imager M2m optical microscope. Figure 4 shows the polished morphology of the cross section of sample No. 2. The inner oxide layer is measured at 2914μm in thickness, while the outer unoxidized tube wall is only 3110μm thick. Figure 5 displays the polished morphology of the inner surfaces of samples No. 2 and No. 4. Sample No. 2 exhibits evident internal oxidation and intergranular corrosion, approximately 60μm deep, whereas sample No. 4 shows no significant internal oxidation or intergranular corrosion characteristics, aside from the surface oxide layer. Figure 6 illustrates the microstructural morphology of the inner surfaces and adjacent areas of samples No. 2 and No. 4. The microstructure of sample No. 2 consists of ferrite, while that of sample No. 4 consists of ferrite and pearlite.
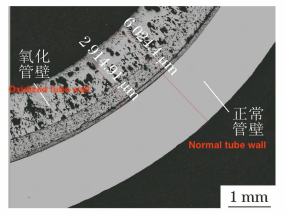
Figure 4 Partial morphology of polished cross-section of sample No. 2
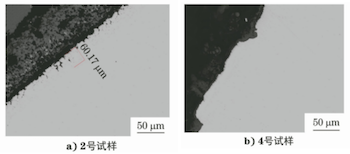
a) Sample No. 2 b) Sample No. 4
Figure 5 Polished morphology of the inner surface of sample No. 2 and sample No. 4

a) Sample No. 2 b) Sample No. 4
Figure 6 Microstructure morphology of the inner surface of sample No. 2 and sample No. 4
The matrix microstructural morphology of Samples No. 2 and No. 4 is shown in Figure 7. The matrix microstructure of Sample No. 2 consists of ferrite with a grain size of grade 8, whereas the matrix microstructure of Sample No. 4 consists of ferrite and pearlite, with a grain size of grade 10.

a) Sample No. 2 b) Sample No. 4
Figure 7 Matrix microstructure morphology of No. 2 and No. 4 sample
The fracture of Sample No. 2 was analyzed using a Stemi508 stereo microscope and a Sigma300 scanning electron microscope (SEM), and its macroscopic morphology is shown in Figure 8. Significant oxidation is observed throughout, and the inside presents a circular platform, which is inferred to be an oxide layer. The outer section rolls up to both sides, likely caused by the explosion of the metal matrix. The crack source is not apparent, but based on the stress conditions of the steel pipe during operation, it can be inferred that the explosion occurred from the inside out. Figure 9a shows the partial SEM morphology of the fracture, divided into Area A (close to the inner surface), Area B (center of the wall thickness), and Area C (near the outer surface). Figure 9b shows the SEM morphology of Area A, which exhibits distinct intergranular features and coarse grains; Figure 9c shows the SEM morphology of Area B, where some intergranular morphology is still visible; Figure 9d shows the SEM morphology of Area C, which is covered by an oxide layer, obscuring the fracture characteristics.

Figure 8 Overall morphology of fracture of sample No. 2
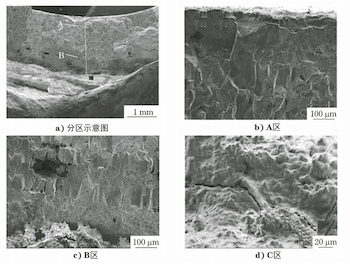
a) Partition diagram b) zone A c) zone B d) zone C
Figure 9 SEM morphology of thefracture of the boiler tube
The blockage inside the tube of Sample No. 4 was collected and ground into powder. Phase analysis was conducted using the XRD-7000 X-ray diffraction (XRD) instrument. The analysis results indicated that the blockage was primarily composed of NaCl, NaCO₃, and other compounds. The analysis results are presented in Figure 10.
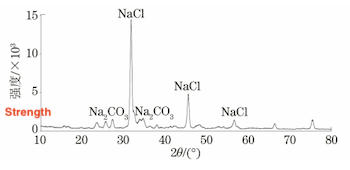
Figure 10 XRD Spectrum of Blockage in the Tube of Sample No. 4
The design drawings indicate that the original wall thickness of the Q245R steel boiler tube is 4.5 mm, and the microstructure of Q245R steel should consist of ferrite and pearlite. Physical and chemical test results show that, compared to Sample No. 4, the inner wall of Sample No. 2 at the burst location exhibits significant corrosion thinning, along with clear internal oxidation and intergranular corrosion characteristics. The microstructure is ferrite, with significantly reduced carbon content, low hardness, and large grain size, indicating abnormal temperature at this location, which caused high-temperature oxidation and severe decarburization of the pipe fittings. The blockages in the pipes that did not burst were primarily composed of NaCl, Na₂CO₃, and other compounds, likely related to impurities in the water vapor. The blocked pipes were located at the bends, where the steam flow rate decreases, allowing impurities to gradually deposit and block the pipes. The steam flow is disrupted, and the temperature is relatively low. There is no decarburization or high-temperature stress corrosion at this location. The burst pipes were the sections located in front of the blocked areas. The rear pipes were blocked and narrowed, causing abnormal steam flow rates and temperature fluctuations. Under exposure to elevated temperatures and corrosive media, high-temperature stress corrosion occurs, making the grain boundaries more susceptible to oxidation, which promotes intergranular fracture.
The high-temperature stress corrosion of the Q245R steel boiler tube likely follows the mechanism of anodic dissolution. The mechanism involves the oxide film on the surface being corroded and damaged under the influence of stress and corrosive media, causing the damaged surface to act as an anode and the undamaged surface to function as a cathode. The metal at the anode ionizes and dissolves, generating a current that flows to the cathode. Because the anode area is much smaller than the cathode area, the current density at the anode is very high, which accelerates corrosion on the damaged surface. This type of corrosion typically exhibits characteristics common to carbon steel.
The medium inside the Q245R steel boiler tube contains a significant amount of NaCl. While NaCl is not directly corrosive, it acts as an electrolyte, increasing the conductivity of the solution and accelerating corrosion. The chemical process is as follows:
Anode reaction:
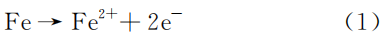
Cathode reaction:
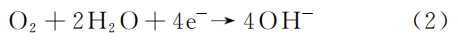
Total reaction:
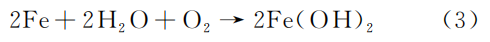
In the presence of sufficient oxygen, Fe(OH)₂ further oxidizes to form Fe(OH)₃, and after dehydration, rust is formed. Based on the physical and chemical test results, it can be concluded that the burst of the Q245R steel boiler tube was caused by NaCl in the water vapor. Numerous impurities, such as Na₂CO₃, gradually deposited at the bends in the pipe, leading to blockages. Due to abnormal steam flow and temperature in the front section of the pipeline, the inner wall of the pipe underwent constant oxidation under high temperature, high pressure, and corrosive conditions. This caused thinning and decarburization of the pipe wall, reducing the original material strength and gradually decreasing the pressure-bearing capacity of the Q245R steel boiler pipe. When the bearing capacity drops to a critical point, the pipe bursts.
The water vapor in the Q245R steel boiler pipe contains numerous impurities, such as NaCl and Na₂CO₃, which cause blockages and narrowing at the bends. This leads to abnormal steam flow rates and temperatures in the front section of the pipe. Due to the effects of high temperature, high pressure, and corrosive media, the inner wall of the pipe undergoes continuous oxidation, thinning, and decarburization. This significantly reduces the strength of the pipe, making it unable to withstand the working pressure, ultimately leading to the pipe bursting. It is recommended to strictly control water quality, reduce impurities in the water vapor, and prevent scale formation in the pipe caused by poor water quality.